Fast & Accurate Mobile COVID Testing
COVID Testing You Can Trust
One Health Labs provides accurate, same-day test results to protect yourself, friends, family, co-workers, and others.

Our Lab Certifications


Services
Get same-day results 24 hours a day with on-site lab testing, pre-travel testing, and screening for special events.
Locations
Convenient locations nationwide. Find a One Health Labs mobile COVID testing location in your area.
Set Up a Lab
Interested in setting up a new lab? Contact a One Health Labs representative to learn how we can set up a new mobile COVID testing lab in your area in as few as 2 weeks.

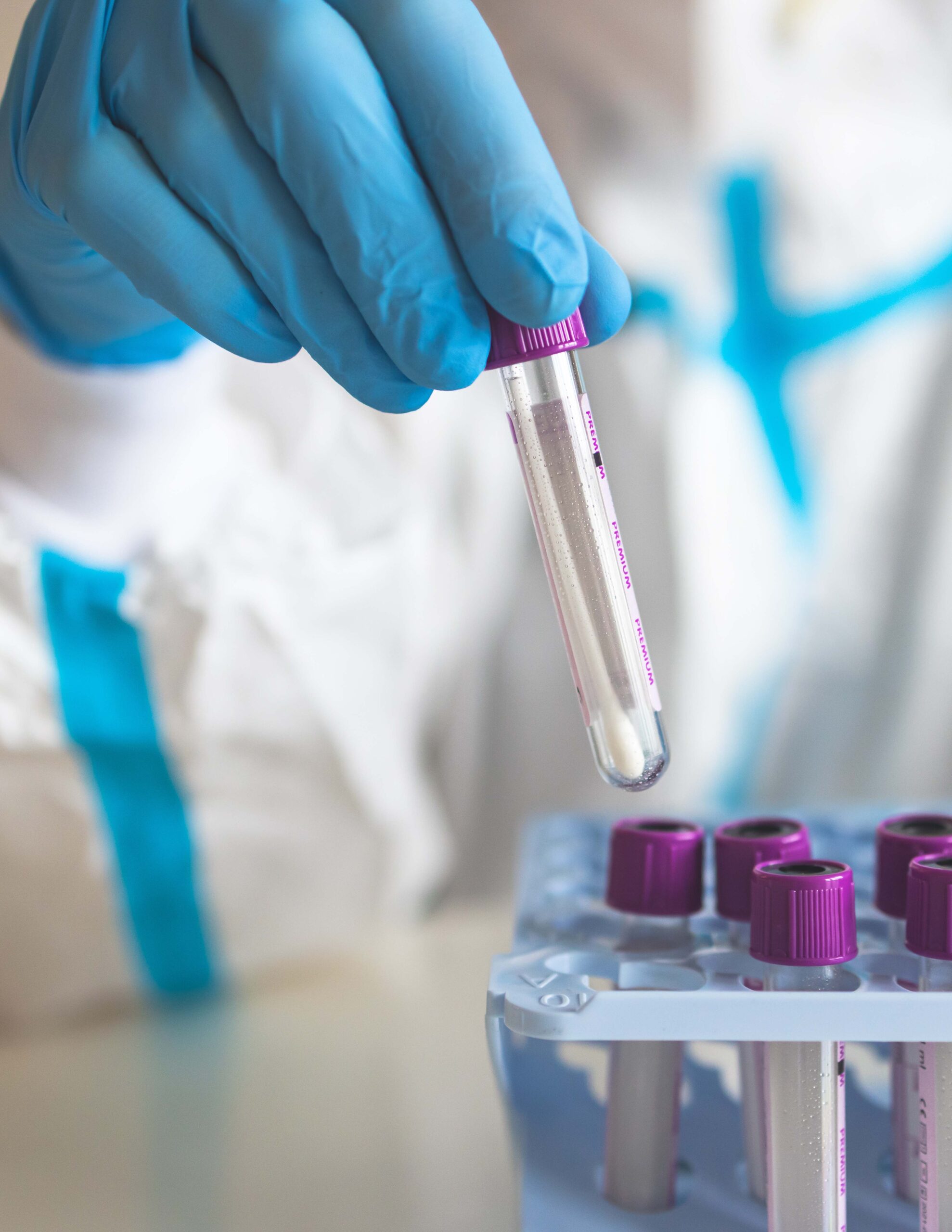
One Health Labs Uses Gold Standard Real-Time RT-PCR For Ultimate Reliability
Trust in your results when you test with One Health Labs. Our mobile COVID testing labs use real-time RT-PCR technology, are authorized under FDA Emergency Use Authorization, and detect all current variants of concern including Delta and Omicron.
Our platform
Your Health,
On Your Time
Who We Serve
Film, TV, & Commercials
Stay on schedule with our SAG-approved, on-site mobile lab testing. We have a nationwide presence and a variety of services to ensure your production team stays safe.
Corporate
Return to the office safely with mobile lab testing from One Health Labs. One Health Labs can set up a weekly lab to screen your team at your office.
Events
Keep your guests safe with One Health Labs event testing. We specialize in same-day turnaround time and concierge testing.
K-12
Keep students, parents, and educators healthy and their schools open with same-day test results.
Universities
Empower students and faculty to safely stay on campus to get the most of higher education with routine, mobile COVID testing.
Pre-Travel
Travel with peace of mind with same-day COVID pre-travel screening. Schedule an appointment or choose one of our walk-in clinics for testing on your schedule.
Why Choose One Health Labs
Guaranteed 12-Hour Turnaround Time
We understand the peace of mind that comes with your results. With results in as few as two hours, we guarantee results within 12 hours.
Flexible Scheduling
Have your plans changed? Do you need to change the location or time of testing? We’ll adapt our plans to fit yours, however and whenever they change.
CLIA-Certified and CAP-Accredited Labs
One Health Labs goes above and beyond when it comes to establishing the highest standard at all of our labs across the country.
Accurate Results
One Health Labs uses gold standard technology in COVID-19 testing, using RT-PCR testing that guarantees results you can trust.





